Ectd Templates has a variety pictures that related to locate out the most recent pictures of Ectd Templates here, and with you can acquire the pictures through our best Ectd Templates collection. Ectd Templates pictures in here are posted and uploaded by Adina Porter for your Ectd Templates images collection. The images that existed in Ectd Templates are consisting of best images and high setting pictures.

ctd dossier preparation k srikantha reddy sr ppt video from Ectd Templates
These many pictures of Ectd Templates list may become your inspiration and informational purpose. We wish you enjoy and satisfied once our best describe of Ectd Templates from our accretion that posted here and as a consequence you can use it for up to standard needs for personal use only. The home Design Ideas team plus provides the other pictures of Ectd Templates in high Definition and Best character that can be downloaded by click on the gallery below the Ectd Templates picture.
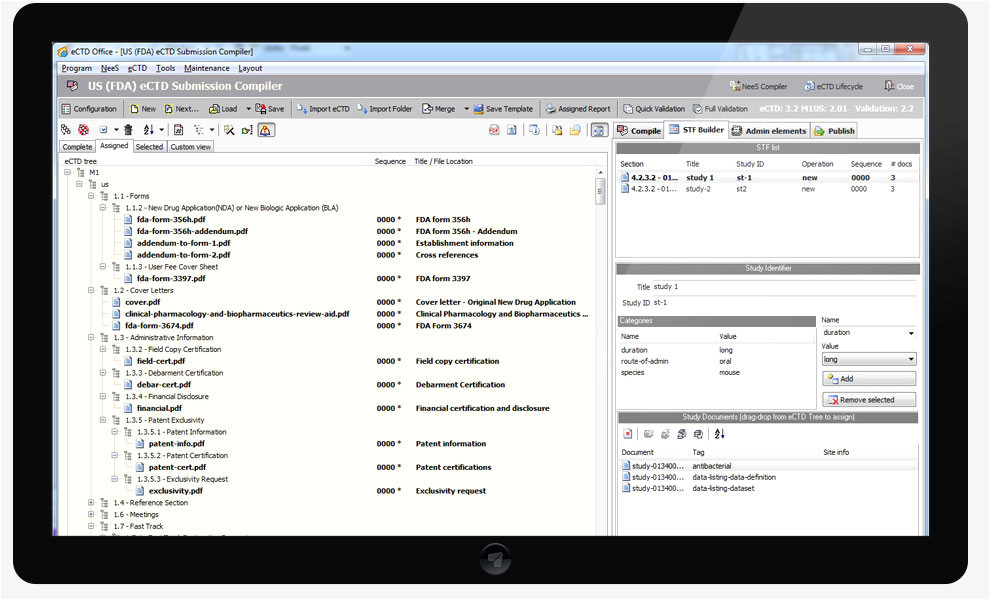
us fda ectd compiler ectd office ectd nees vnees from Ectd Templates
ectd submissions from Ectd Templates
You Might Also Like :
[gembloong_related_posts count=3]
williamson-ga.us can urge on you to acquire the latest guidance very nearly Ectd Templates. improve Ideas. We present a top feel tall photo with trusted allow and all if youre discussing the house layout as its formally called. This web is made to position your unfinished room into a comprehensibly usable room in comprehensibly a brief amount of time. therefore lets consent a enlarged judge exactly what the Ectd Templates. is anything virtually and exactly what it can possibly accomplish for you. considering making an ornamentation to an existing domicile it is hard to build a well-resolved development if the existing type and design have not been taken into consideration.

dia ers siac ind cmc ectd submissions part ii ind to nda from Ectd Templates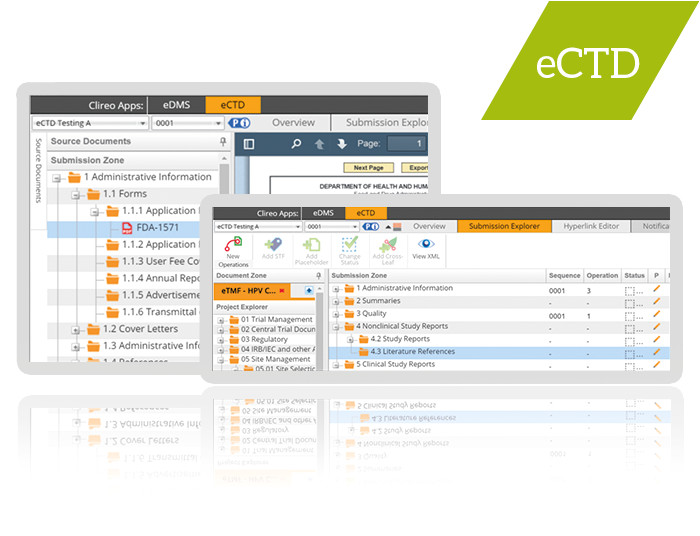
ectd publishing software and services clireo ectd from Ectd Templates
sage templates for ectd sage submissions sage submissions is dedicated to helping you streamline the process for creating submissions for drug biologic and medical devices sage templates a ms word based tool helps you create documents that support the global electronic common technical document ectd submission standard ectd software the electronic common technical document ectd developed by the international conference on harmonisation ich is now the standard format for the submission of electronic regulatory documents in the us canada europe and japan registrations ectd publishing software mastercontrol com registrations ectd publishing software for pharmaceutical and biotech companies product registration and regulatory submissions requirements in different countries is a time consuming and nerve wracking process ectd software fda ectd publishing tool demo vendor freyr submit is a web based cloud hosted on premise ectd software tool which helps life sciences companies in creation validation publishing viewing and reporting for regulatory document management for electronic submissions to comply with fda ema health canada gcc sfda tga mccza swiss medic cfda moph etc key considerations for the upcoming mandatory ectd april 27 2017 kathy elks principal consultant and jeff golden associate director regulatory operations people who work in regulatory affairs and especially those in regulatory operations are well aware of the fact that it is less than a month before ectd submissions will become mandatory ectd validator electronic submissions globalsubmit validate used exclusively by us fda ectd validator electronic submissions checks for 200 errors multiple regions supported validation reports web based heads of medicines agencies procedural guidance c heads of medicines agencies http www hma eu 27 html heads of medicines agencies esubmissions requirements on submissions number and format for new ma applications within mrp dcp or national procedures november 2018 track version globalsubmit software and regulatory services for ectd submissions management globalsubmit s submissions management suite consists of solutions regulatory operations professionals need to efficiently publish validate and review ectd submissions plus manage a solution to tie submissions to the bigger picture of regulatory information management home regulatory affairs and quality assurance antrix antrix offers full range of expert regulatory affairs and quality assurance consulting services to the ivd medical device pharmaceutical and biotech industries worldwide
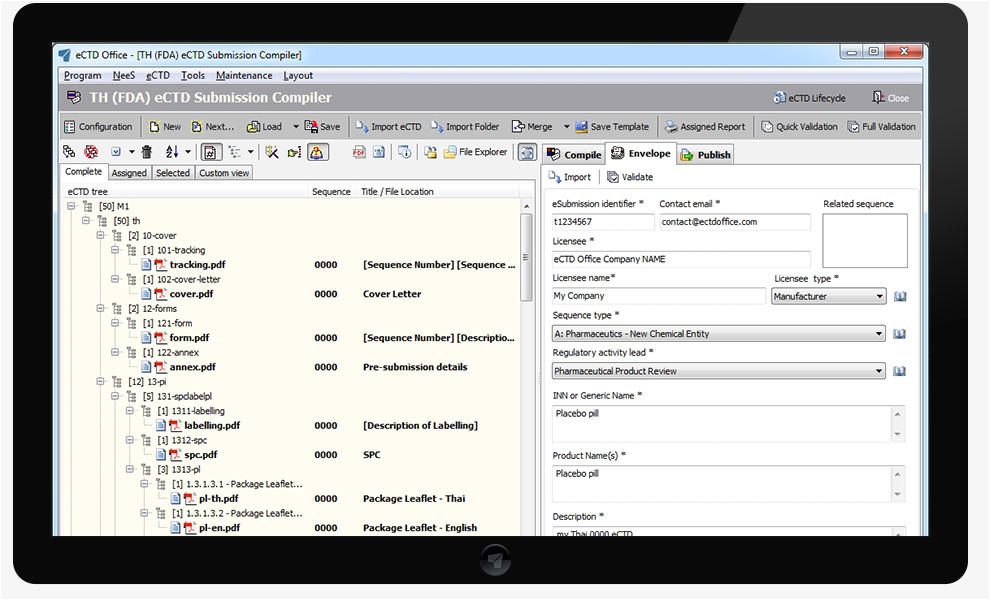
gcc ectd compiler ectd office ectd nees vnees from Ectd Templates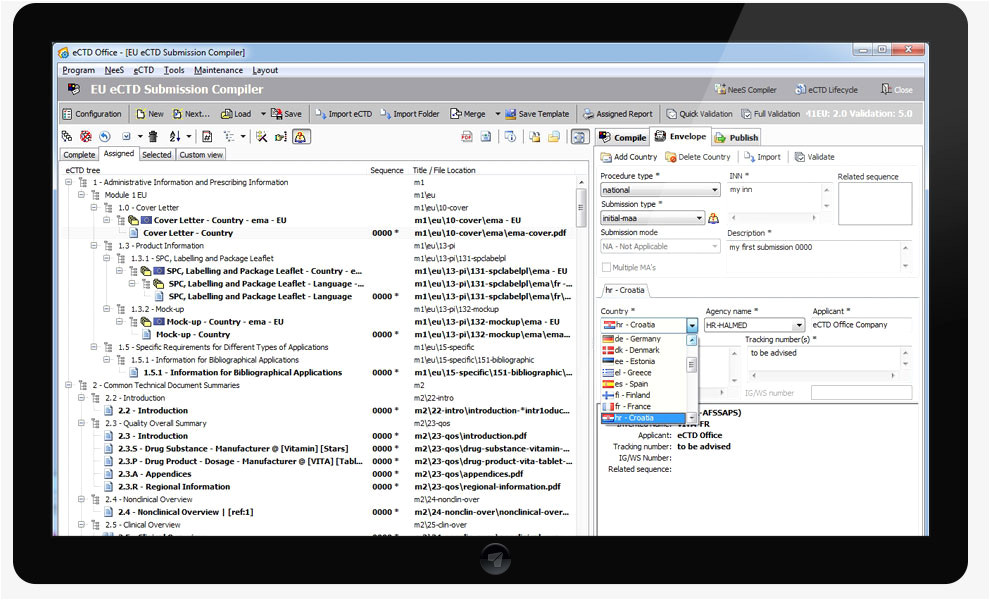
eu ectd compiler ectd office ectd nees vnees from Ectd Templates
ectd format narsu ogradysmoving co from Ectd Templates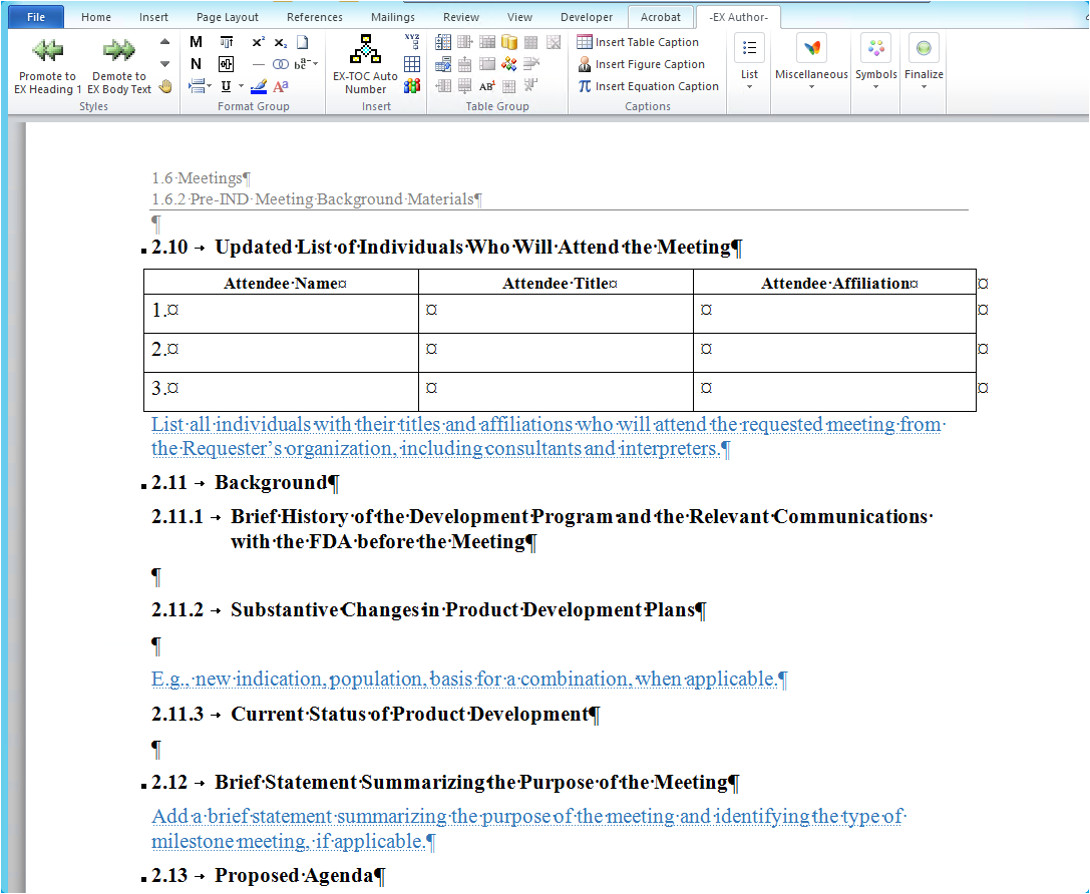
ectd publishing esubmission software extedo ectdmanager from Ectd Templates